Solubility Rules
Solubility is defined as the amount of solute that can dissolve in a given quantity of solvent. Generally speaking, the higher the solubility, the greater the amount of solute that can be dissolved in a solvent and vice versa. While these definitions may seem quite straightforward on the surface, it's beneficial to adopt standard guidelines when discussing solubility in order to ensure all parties are working with a shared understanding. The United States Pharmacopeia (USP) has created exacting definitions for this purpose – ones which are adopted by other major pharmacopeia organizations around the globe. These definitions lay out measurements and other details which can aid accurately in relating solubility to operational needs.
Solubility Effects on Reactions
When a solution becomes saturated with a solute, each subsequent addition of more of that component leads to a decrease in its concentration as the excess is forced out and converted into a precipitate. This process, called precipitation, can affect the overall energy of any reaction occurring in that system as well as cause changes in electrical conductivity. As a result, solubility effects have to be taken into account when designing chemical reactions in order to ensure that their outcome is predictable. Furthermore, insoluble components or precipitates can often be found at the end stages of reactions and determine the form and purity of the product.
Solubility chemistry
Solubility chemistry is a critical part of understanding how chemical compounds interact. There’s a wide range of solutes – from those that dissolve completely, to those that form precipitates or solids, to those that react with water in some way. When making certain solutions and compound mixtures, it’s essential to consider these factors in order to ensure that the intended outcome will be achieved. Understanding the impacts of solubility on outcomes can help chemists avoid costly mistakes or unexpected results in their experiments.
What is Solubility?
Solubility is the measure of how much solute can dissolve in a solvent. Commonly, this is expressed as grams of solute per liter of solvent. For example, “insoluble” substances have less than 1 gram dissolving in a liter, while “soluble” substances have more than 30 grams dissolve in a liter. Depending on how much solute is dissolved into the solvent, you will often get events such as precipitates being formed or saturated solutions with no room for extra solute. Coffee filters are a great way to capture these separate compounds and leave a clear solution behind. In certain cases, it is even possible to supersaturate solutions by increasing its temperature to make more room for the solute before slowly cooling down the mixture so vapor pressure decreases and make an excess amount of solute be included in the solution.
Solubility Rules Chart
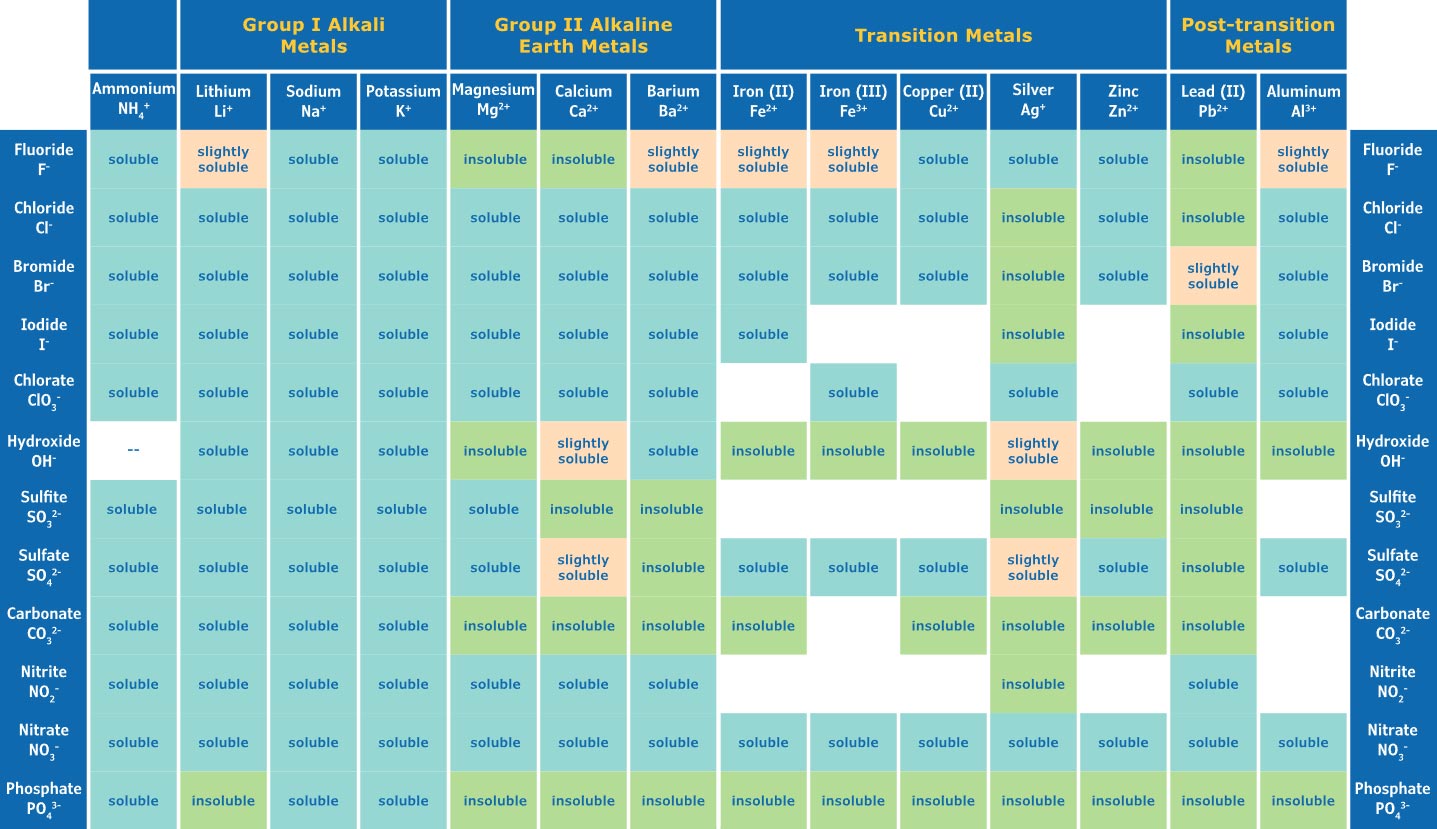
Solubility Rules
If you want to determine the solubility of a compound, start by writing out its empirical formula. This will help you identify each of the ions that make it up. You can then use either solubility rules or a solubility chart to check the compound's solubility. With the rules, look in the left-hand column for the general rule and check any exceptions listed in the right-hand column. To use the chart, identify your cations across the top and anions vertically, and locate where those two meet to find your answer. If you are dealing with uncommon compounds, you may need to consult a periodic table for further illustration.
Understanding solubility rules can help to make sense of the behavior of anions and cations in water. Generally speaking, salts of alkali metals plus NH4+ are usually soluble in water, whereas nitrates (NO3−), acetates, chlorates and perchlorates are always soluble. Chlorides, bromides and iodides are generally soluble with the exception of silver compounds, with the exception of silver nitrate and silver acetate being exceptions that prove the rule! On the other hand, sulfates are normally soluble as well, apart from those that contain Ca+2, Sr+2, Ba+2 and Pb+2. Hydroxides also exhibit opposite behavior to what we might expect - with alkali metal salts being totally soluble and those containing Mg+2, Ca+2 Sr+2 and Ba +2 being slightly so. Understand these small variances in behavior between the ions is critical when thinking about particles interacting in aqueous solutions.
- Solubility rules are guidelines that chemists use to predict whether a given compound will be soluble in water
- The most important solubility rule is that "like dissolves like." This means that compounds with similar chemical properties will tend to be soluble in each other, while compounds with dissimilar chemical properties will tend to be insoluble in each other
- Another important solubility rule is that polar compounds are generally soluble in water, while nonpolar compounds are generally insoluble in water
- There are a few exceptions to the rule that polar compounds are soluble in water. For example, some ionic compounds (compounds consisting of ions) are insoluble in water
- The solubility of a given compound can also be affected by temperature. In general, compounds tend to be more soluble at higher temperatures and less soluble at lower temperatures
- The solubility of a given compound can also be affected by pressure. In general, gases are more soluble at higher pressures and less soluble at lower pressures
- The final solubility rule is that the solubility of a given compound can be increased by the addition of a solvent with a similar chemical structure
- There are two main types of solvents: polar and nonpolar. Polar solvents have a dipole moment, meaning that they have an uneven distribution of electrons. Nonpolar solvents do not have a dipole moment
- Water is the most common solvent, and it is considered to be a universal solvent because it can dissolve both polar and nonpolar compounds
- Other common solvents include alcohols, ketones, and acetic acid